Hot Ice

'Hot Ice' is when we grow a crystal of sodium acetate from a solution, and doing so we release heat!
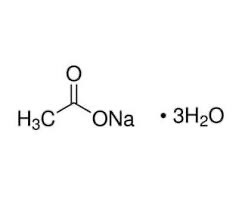 Chemical structure of sodium acetate trihydrate
Chemical structure of sodium acetate trihydrate
This ability to release heat during crystal formation, makes it useful for the reusable hand warmers/heating pads.

How does it work?
Sodium acetate trihydrate crystals melt at 58 °C. The melting is the removal of the tree molecules of water (= thihydrate) incorporated into the crystal, in whitch the salt disolves, creating a supersaturated solution. It is then possible to cool this solution without the formation of crystals to the room temberature or even lower!
The crystal will start to grow from a nucleation centre, as on the photo below. The nucleation center can be started by putting an object (knife, toothpick, cocktail stick, ...) into the solution or, in the case of handwarmer, a click of the metal disk in the sealed within. As the crystal of sodium acetate trihydrate grows radially across the container, picking up the waters from solution, the heat is released (exothermic reaction).
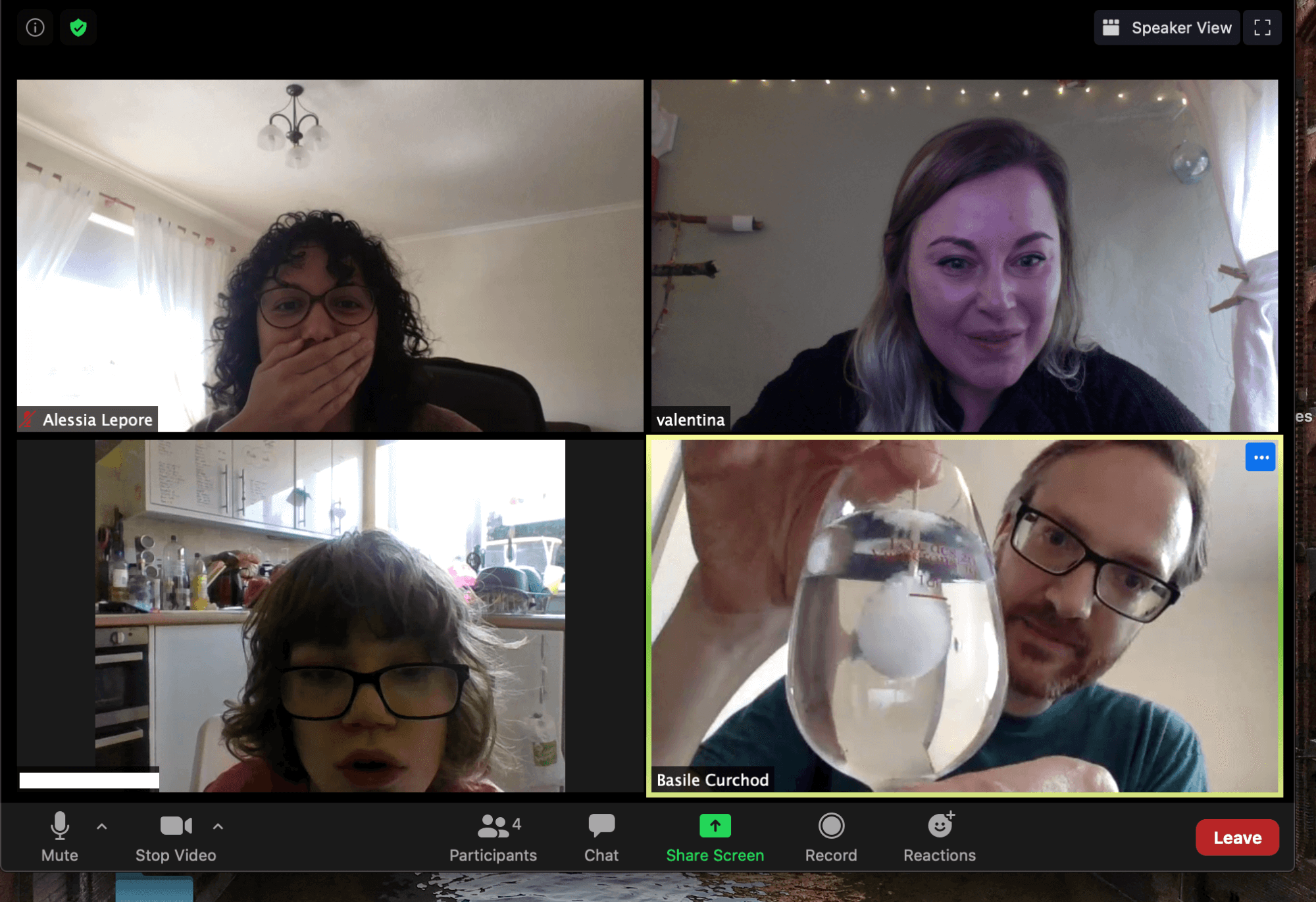 Basile demonstrating the 'hot ice' nucleation during a call
Basile demonstrating the 'hot ice' nucleation during a call
This reaction is reverseable, and so we can reheat the crystals, melting waters away and disolving sodium acetate in them.
Try experiment at home:
You will need:
- 300g of sodium acetate crystals (can be purchaced online)
- mixing jug
- very clean glass without scratches
- cocktail stick
Method:
- Measure 300g of sodium acetate
- Pour 80ml (5 tablespoons of water for the reactive version) of water into a mixing jug and mix in the crystals of sodium acetate.
- Microwave the mixture for 10-20 seconds and stir it with a metal spoon, repeat as necessary until the solution is perfectly clear (everything is dissolved).
- One everything is dissolved, pour the solution carefully into a very clean glass. Pro Tip 1: first rinse the glass with warm water inside, it will keep it warm and clean.
Pro Tip 2: keep the pan as crystals will form at the bottom and can be used to seed the crystallisation process. - Place the glass with sodium acetate solution into the fridge for at least 2 hours (until the solution is cold).
- Take the glass from the fridge and use a cocktail stick to trigger the crystallisation (if it doesnt work you may need to put a little crystal from the pan onto the cocktail stick and submerge it).
- You can reuse the formed crystal! Just pop it into a microwave to heat up, stir it a little bit and place into a new glass (rinced by warm water)!
- Share your videos and pictures with us via Twitter @Scientist_ND and Facebook @scientistND