Lemon Battery
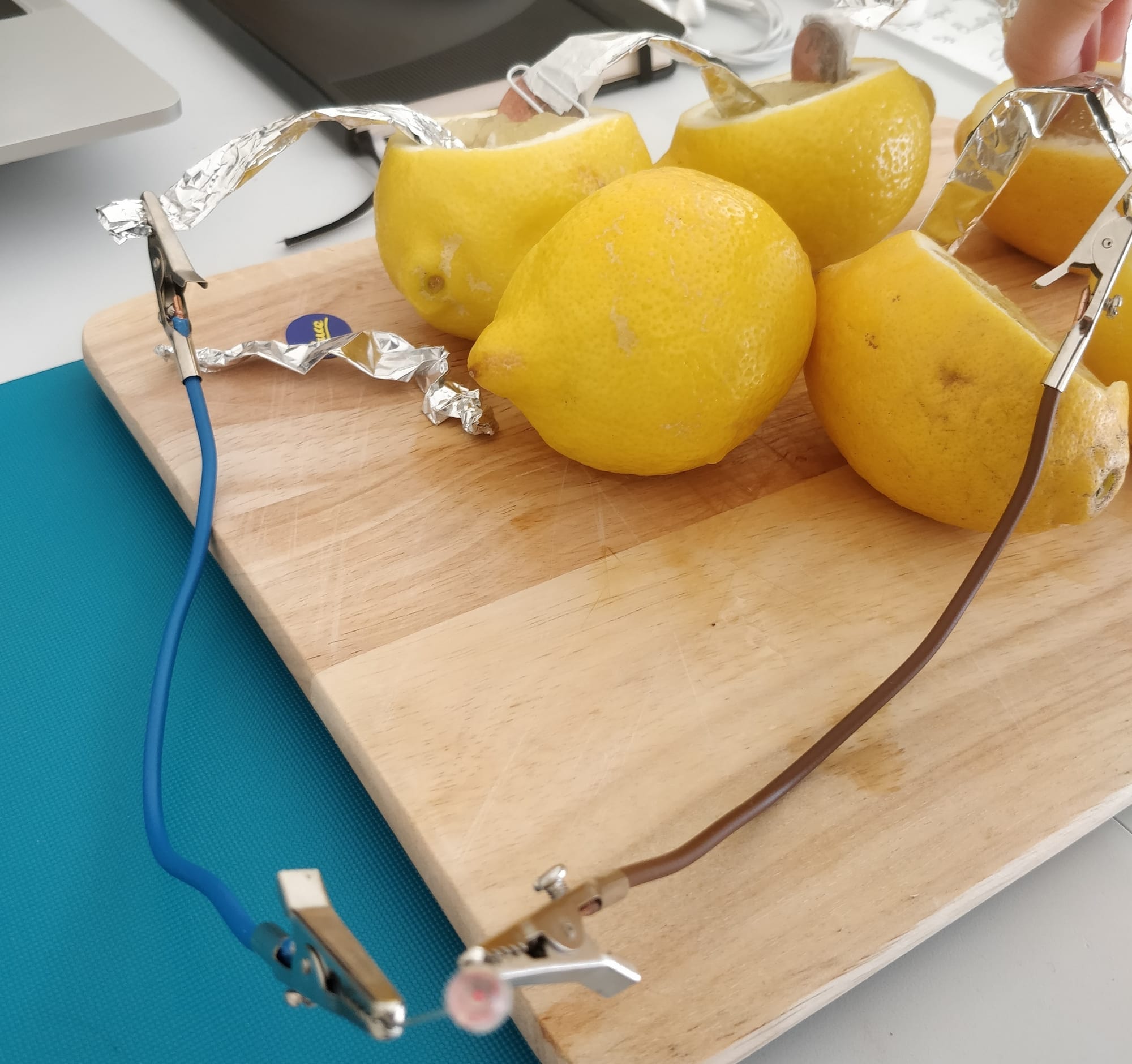
🍋This is an experiment to demonstrate how lemony chemical energy can be turned into electrical energy!
🍋This lemon battery is based on a similar concept as the very first battery, built by Alessandro Volta in 1799!
🍋The battery works by moving electrons (little charged particles) from anode (negative: zinc metal, paper clip or a galvanised nail) to cathode (positive: copper metal, e.g. a penny coin) via the fruit.
🍋If you set up your circuit correctly, you will be able to see it powering a small LED light.
🍋Instead of lemons, you can also use other citruses (limes, oranges, grapefruits), or other acidic fruits. You can also use potatoes, they have a different chemical (phosphoric acid instead of citric acid) that allows them to power the battery.
🍋To make this battery work better, you can roll the fruit by such making it juicier inside or, if using a potato, part boil it.
🍋You can also use acidic liquids, such as vinegar (acetic acid) instead of lemons.
🍋The more lemons/fruit (AKA battery cells) you hook up, the more powerful this battery will be!
Here is a photo from Hope set up by Alessia, that she then demonstrated on a call!
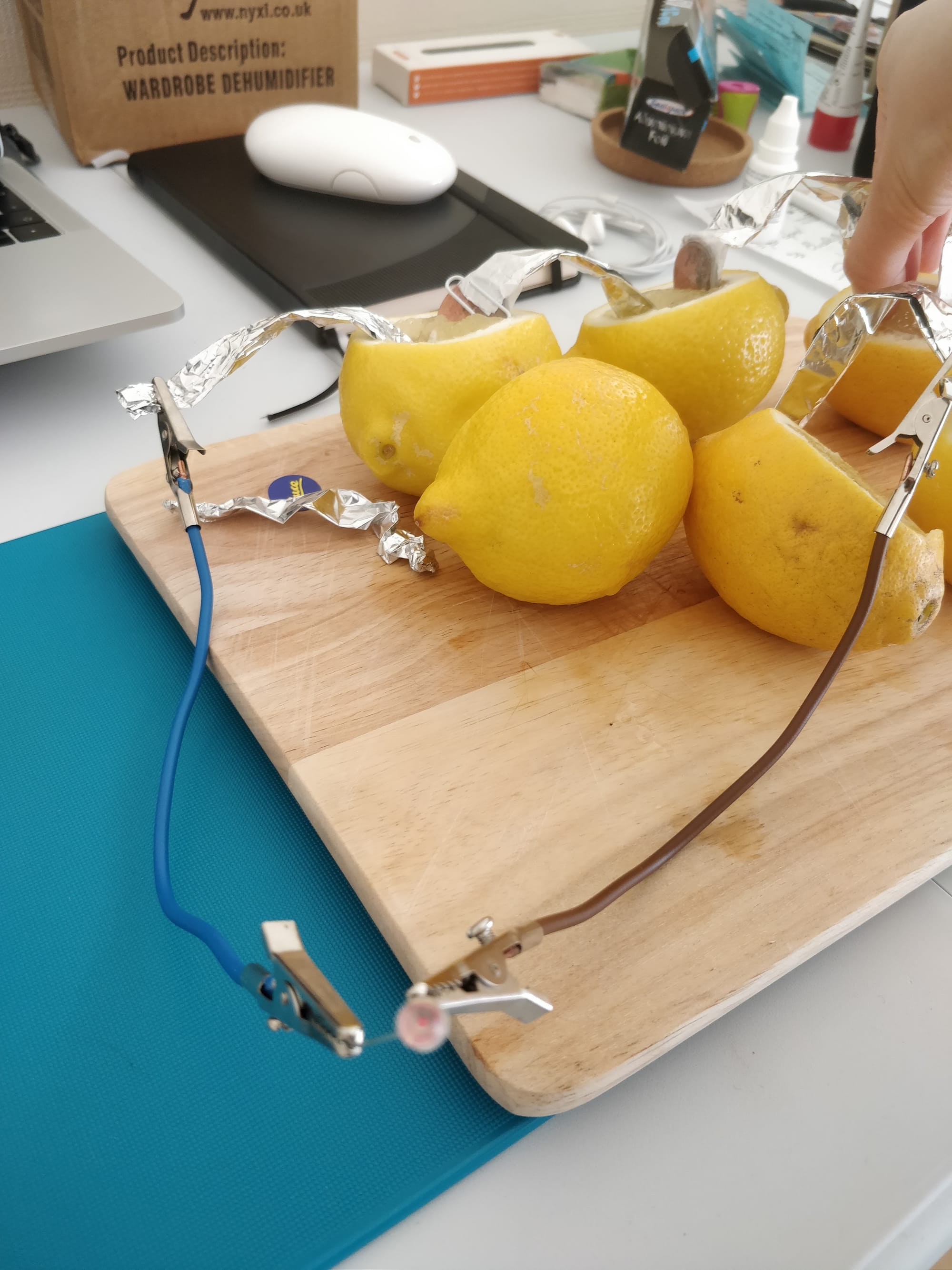
And here is a PDF of the experiment - please read it before doing the experiment!
So, which makes a better battery: 🥔POTATOES or 🍋 LEMONS? Here is a video!
🍋🍋🍋🍋🍋🍋🍋🍋🍋🍋🍋🍋🍋🍋🍋🍋
And this is currently the BIGGEST lemon battery!