Injectable 3D Scaffold and Bone Tissue Engineering
What is the safest bone grafting material that is injectable, biodegradable, biocompatible, cross linking in situ (provide fit contact with the bone defect), and in the same time has mimic properties of ideal bone with easier manipulation, less cost and less complication ? Bone scaffold is in high demand in dentistry and orthopaedic surgery for regenerating osseous defects. To minimize the dental procedures and maximize the quality of bone healing, a bioactive injectable and in situ cross-linkable compound development that is easily implanted, able to fill irregular bone socket, as well as growth factors and osteogenic cells carriers is essential. This kind of biomaterial has the potential to provide a good future for implant, bone defect treatment, and cosmetic applications by alveolar bone preservation with lesser side effect.
Our main was to develop new 3D scaffold that has a major advantage of other injectable material in that it can be injected directly into the bone defect site and then set in situ at room temperature. Other alternative material needs to be preheated to a high temperature before it can be injected into any osseous defect site which sequentially resulted in necrosis or damage to the native tissue. Furthermore, its degradation process can take up to two years, which is a significant drawback clinically.
To overcome this problem, our objectives were to develop a novel injectable, in-situ self-crosslinkable at a room temperature, biodegradable polymeric scaffold based on gelatin microparticles porogen, named as poly(caprolactone-trifumarate)-gelatin microparticles (PCLTF-GMPs) for bone tissue engineering applications. Secondary objectives were to evaluate the biocompatibility, characterisation, and mechanical stability of this scaffold in vitro and study its behaviour in vivo.
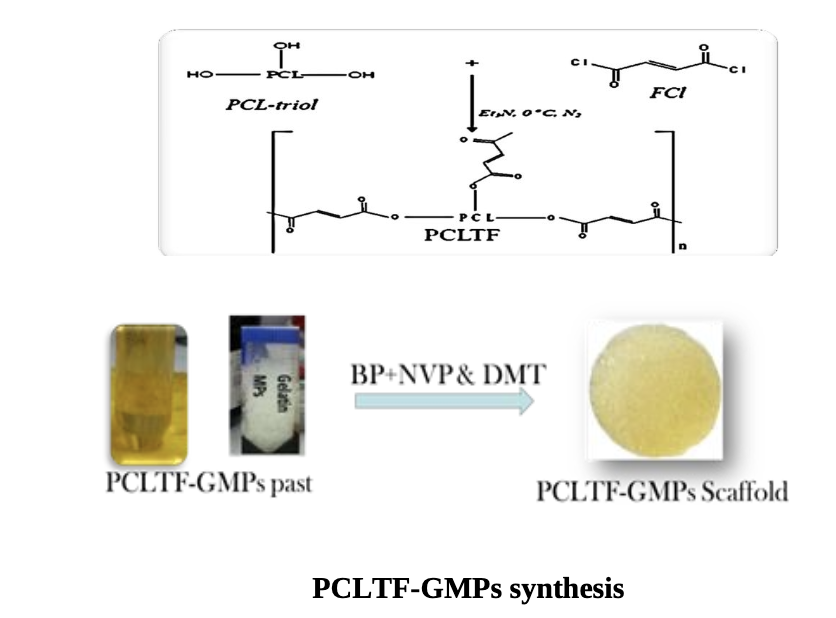
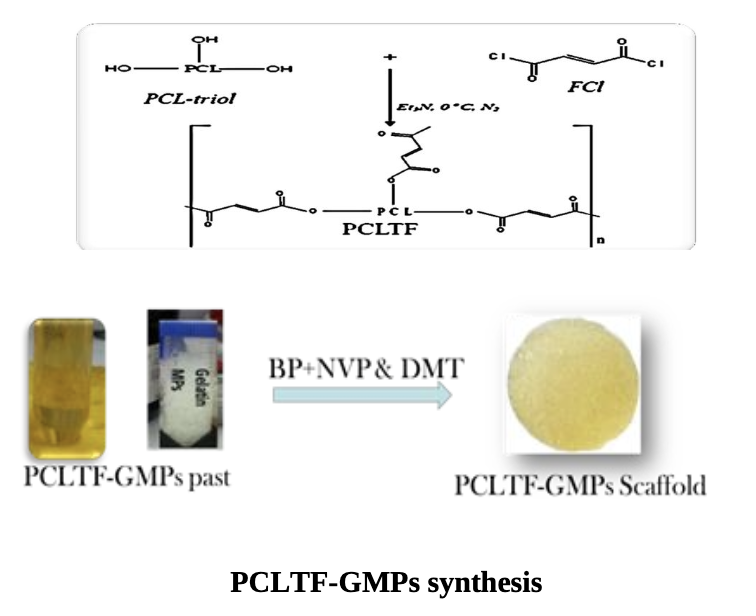
The PCLTF was synthesized via the chemical reaction between PCL-triol and FCl, with K2CO3 acting as catalyst. Fourier transform infrared spectrometry (FT-IR) and Nuclear magnetic resonance (1H-NMR) spectrum were used to confirm the presence of PCLTF. This PCLTF was combined with crosslinking agents and gelatine microparticles (GMPs) porogen to produce a three-dimensional (3D) porous scaffold structure. Topological features of the PCLTF- GMPs scaffold and its surface roughness at both micron and nanometer scales proposed to influence the cell attachment and proliferation. Then human fibroblast and osteoblast cells proliferation on the surface of PCLTF- GMPs scaffold was measured and examined under field scanning electron microscopy (FESEM). Quantification of cell viability with regards to the leachable extracts and degradation products of the scaffold was evaluated and scanned under fluorescence microscope. It indicated that the Cells treated with scaffold’s products revealed evidence of cell growth, with intact cell morphology and nucleus.
Selected thermal, physical, and mechanical characterizations of the scaffold were evaluated in vitro. The result showed that the mechanical properties of the PCLTF-GMPs was stable and it can potentially be used in non-stress bearing area for procedures involving alveolar bone augmentation, sinus lifting, and non-loading implant insertion. The in vivo behaviour of PCLTF-GMPs was studied in a critical-size bone defect in animal model and the bone remodelling process was analysed. Bythat, its biocompatibility and osteoconductivity has approved through the bone healing and formation of new bone in the PCLTF- GMPs filled critical size cranial defects of rabbits.
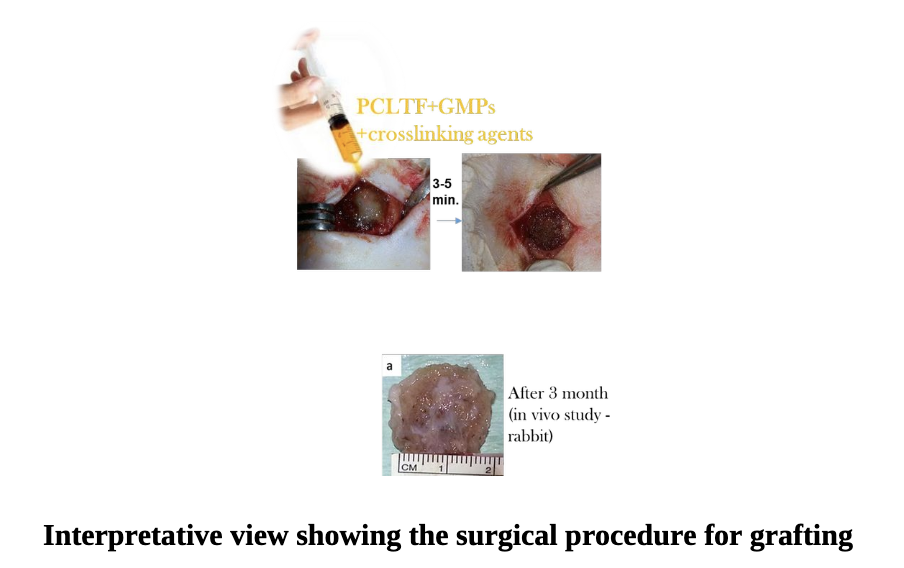
Overall, an interesting candidate material with reliable properties for using as a scaffold for bone repair and reconstruction has been produced. It has a major advantage in that it can be injected directly into the bone defect site and then set in situ at room temperature. It verified its biocompatibility and ability to aid in bone healing in vivo. We are strongly believed that PCLTF-GMPs has the potential to be used as biocompatible as well as in-situ crosslinkable bone filler to treat critical bone defect, periodontal bone defect and alveolar bone augmentation. Infuture, this scaffold can be implanted with osteoblasts cells, growth factors, or PCLTF-based composite scaffolds to enhance its activity (1).
Reference:
1. NM Al-Namnam et al. (2017). An injectable poly(caprolactone trifumarate-gelatin microparticles) (PCLTF-GMPs) scaffold for irregular bone defects: Physical and mechanical characteristics. Materials Science and Engineering: C, 72(1):332-340